This site is for US healthcare professionals only

Albuminuria Is Associated With Increased CV and Renal Risk and Is an Important Component of Early Identification and Diagnosis of CKD Associated With T2D1
UACR is the preferred test to measure albuminuria1,3
UACR is a predictor of CV mortality, independent of eGFR4
Independent of each other and traditional risk factors, UACR ≥10 mg/g and eGFR <60 mL/min/1.73 m2 are significantly associated with increased CV mortality
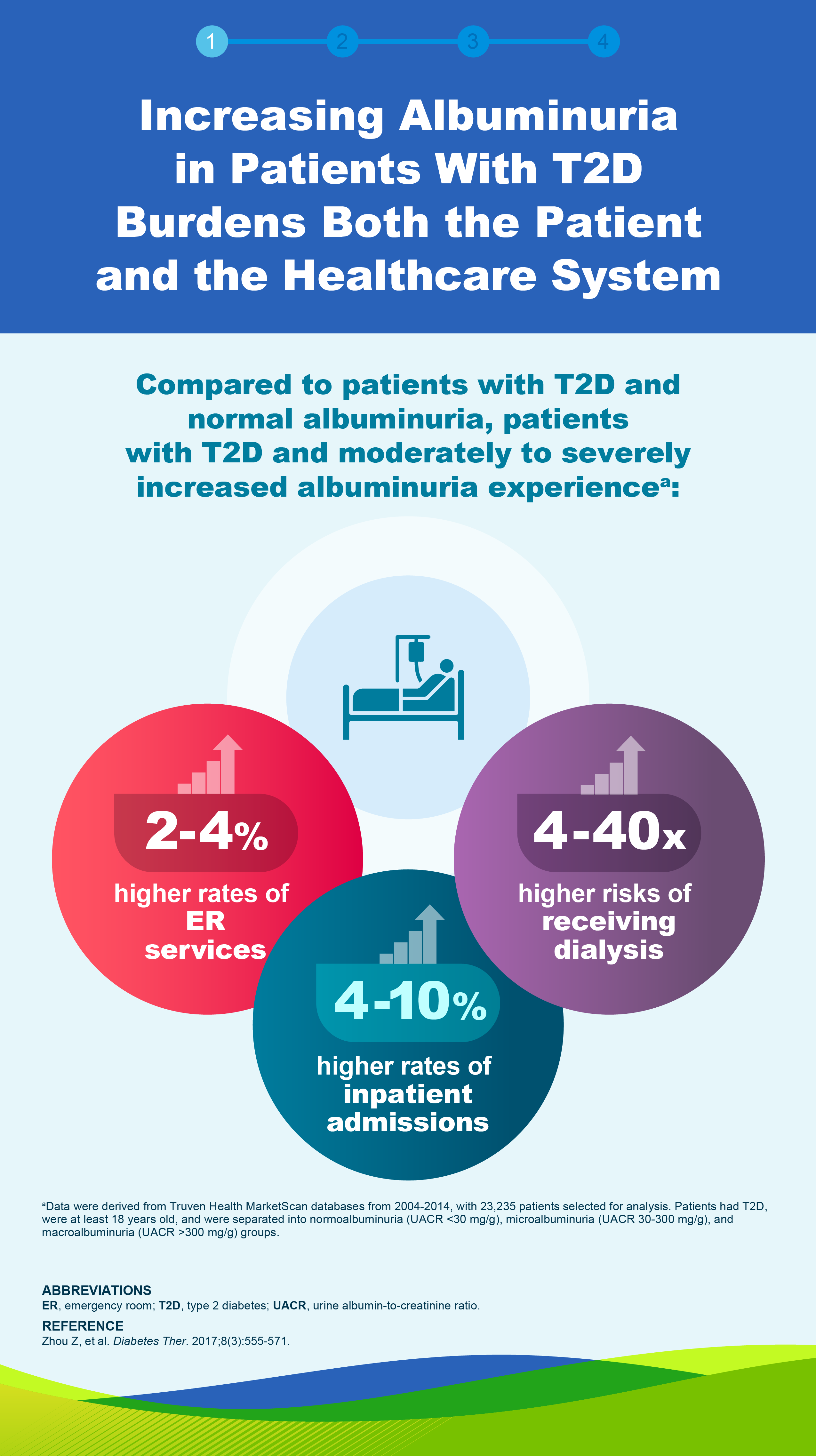
Albuminuria in patients with T2D is associated with kidney disease progression6
5-Year Probability of CKD Progression in Patients With T2Db
Reduction of albuminuria is associated with reduced risk of ESKD in patients with CKD7,c
Want to see more?
Keep Exploring
aAdjusted for each other (UACR or eGFR), age, gender, race, CVD history, systolic blood pressure, diabetes, smoking, and total cholesterol. Circles represent statistically significant and triangles represent not significant.4 bObservational cohort study of 31,931 patients with diabetes and 33,201 patients without diabetes.6 cBetween July 2015 and June 2018, data from 28 cohorts in the CKD-PC were analyzed for this study, which included 693,816 individuals (557,583 [80%] with diabetes). Data for 675,904 individuals and 7461 ESKD events were available for the primary outcome analysis.7
ADA, American Diabetes Association; AER, albumin excretion rate; CI, confidence interval; CKD, chronic kidney disease; CKD-PC, Chronic Kidney Disease Prognosis Consortium; CV, cardiovascular; CVD, cardiovascular disease; DP, dipstick proteinuria; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; HR, hazard ratio; KDIGO, Kidney Disease Improving Global Outcomes; T2D, type 2 diabetes; UACR, urinary albumin-to-creatinine ratio; UPCR, urine protein-to-creatinine ratio.
1. American Diabetes Association. Section 11. Diabetes Care. 2024;47(Suppl 1):S219-S230. 2. de Boer IH, et al. Diabetes Care. 2022;45(12):3075-3090. 3. Kidney Disease Improving Global Outcomes. Kidney Int Suppl. 2013;3(1):1-150. 4. Matsushita K, et al. Lancet. 2010;375(9731):2073-2081. 5. Matsushita K, et al. Supplementary Appendix. Lancet. 010;375(9731):2073-2081. 6. Nichols GA, et al. BMC Nephrology. 2020;21:167. doi: 10.1186/s12882-020-01792-y. 7. Coresh J, et al. Lancet Diabetes Endocrinol. 2019;7(2):115-127.
You are encouraged to report side effects or quality complaints of products to the FDA by visiting www.fda.gov/medwatch or calling 1-800-FDA-1088. For Bayer products, you can report these directly to Bayer by clicking here.